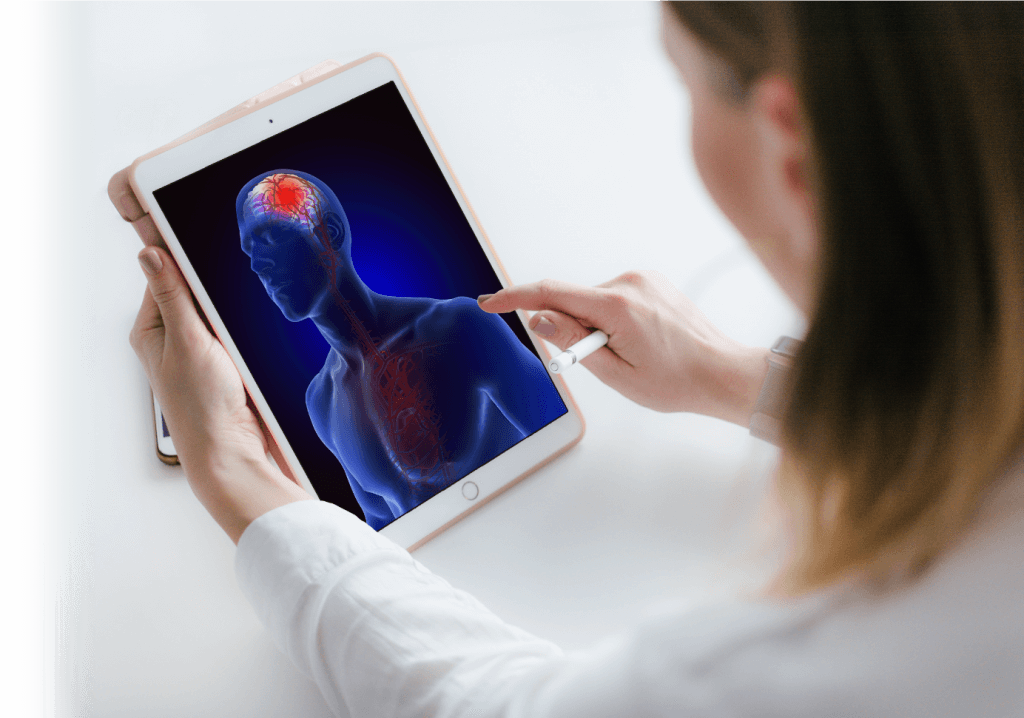
Brain cancer and more generally diseases affecting the brain currently have limited treatments options. The impermeable blood-brain barrier (BBB) hinders therapeutic agent delivery. Finding a new and effective strategy to increase the delivery of existing and novel agents to the brain would revolutionize treatment of these diseases.
The BBB is an impermeable cellular structure that protects the brain from pathogens circulating in the blood but also prevents therapeutic agents from reaching their target (e.g. disseminated tumor cells in normal brain tissue). As a consequence, the BBB limits the efficacy of traditional chemotherapies and of other newer classes of agents such as immunotherapies, which have shown significant clinical efficacy for a broad range of other solid tumors.
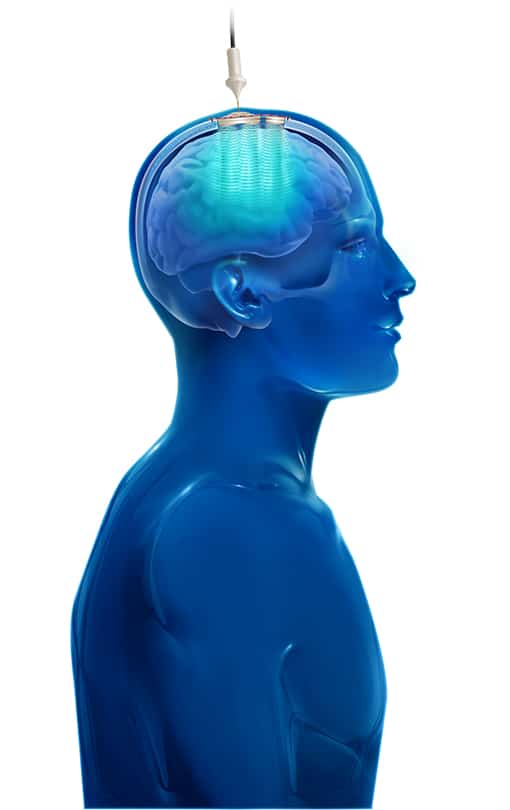
The SonoCloud® device uses Low Intensity Pulsed Ultrasound (LIPU) energy to temporarily disrupt the BBB in a controlled manner. The pulsed ultrasound generated by the device (sonication) creates oscillation of concomitantly infused gas microbubbles (ultrasound resonator). This physical effect induces local and reversible opening of the BBB. Administration of the drug close to the sonication procedure allows for enhanced drug diffusion in the targeted areas of the brain.

This innovative technology […] opens both the BBB, as well as exciting therapeutic possibilities for gliomas, a disease beyond reach for most drugs

Prof. Roger Stupp
Chief of Neuro-Oncology – Department of Neurology at Northwestern University – Feinberg School of Medicine
 GLIOBLASTOMA
GLIOBLASTOMA 
 en
en  fr
fr